English
ESMO Checklist: Pancreatic Cancer Patient Related Treatment Workflow
Tick the box and insert the date as you have dealt with every task listed below, as appropriate. In case you use the template, you can also insert and save data directly on the PDF file.
Download it HERE and send us at segreteria.profboggi@ao-pisa.toscana.it

Contact
International center for pancreatic surgery
We have been on the cutting edge of pancreatic surgery for over 30 years.
We have pioneered surgery of borderline resectable pancreatic cancer and locally advanced pancreatic cancer. We have the largest experience in the world with pancreatectomy including resection and reconstruction of the superior mesenteric artery. In addition to that, we have performed more robotic pancreatic resections (including pancreatoduodenectomy) than any other center in Europe.
PROFESSOR UGO BOGGI, MD, FEBS, has lead the center for 25 years.
Ugo Boggi is Professor of Surgery at the University of Pisa (Italy), where he heads the Division of the General and Transplant Surgery, and Adjunct Associate Professor of Surgery at the University of Pittsburgh (USA). Professor Boggi is a world-renowned, gastrointestinal and transplant surgeon with a strong expertise in the surgical treatment of hepato-biliary and pancreatic cancers as well as in pancreas and renal transplantation. Dr. Boggi practices state-of-the-art robotic surgery having performed the first world robotic pancreas transplant, the first world robotic distal selective spleno- renal shunt for the treatment of severe portal hypertension, and the first European robotic renal transplantation. Dr. Boggi has one of the world’s largest experiences in robotic pancreatic surgery, including robotic pancreatoduodenectomy, and in extended pancreatectomies with resection of both peripancreatic veins and arteries.
Dr. Boggi has the largest experience in the world with pancreatectomy including resection and reconstruction of the superior mesenteric artery. According to Scopus (https://www.scopus.com/authid/detail.uri?authorId=7006650849), as of August 2023, Professor Boggi published 456 peer-reviewed articles and received 13,183 citations, leading to an H-index of 58.
Professor Boggi also published two books
(https://link.springer.com/book/10.1007/978-88-470-3958-2)
(https://www.edizioniets.com/scheda.asp?n=88-467-0474-6), and over 60 book chapters.
Nearly all publications deal with pancreatic disease and pancreatic surgery. For a full list of publications see https://pubmed.ncbi.nlm.nih.gov/ and search for “Boggi U”. Professor Boggi presented invited lectures to >500 National and International meetings. Professor Boggi is Editor-in-Chief of Updates in Surgery (https://www.springer.com/journal/13304/editors), founded the European Journal of Transplantation (https://www.eujtransplantation.com), is member of the Editorial Board of 6 international scientific journals, and servers as a reviewer for over 40 international scientific journals. Professor Boggi is member of National and International Surgical Societies. Currently, he is president of the Italian Society for Organ and Tissue Transplantation (SITO). He was member of the council of the Italian Society of Surgery (SIC – founded in 1882), of the Italian Society of Surgical Oncology (SICO), of the Italian Association for the Study of the Pancreas (AISP), and the Interregional Association for Transplantation (AIRT). Professor Boggy is President of the 125th National Congress of the Italian Society of Surgery (SIC).
Professor Boggi organized the first world consensus conference on pancreas transplantation (Pisa, Italy, October 18-19, 2019), was member of the steering committee of the first world state of the art conference on minimally invasive pancreatic surgery (Sao Paulo, Brazil, April 20, 2016) and of the first internationally validated European guidelines meeting on minimally invasive pancreatic surgery (Brescia, Italy, September 29-30, 2022), and was a faculty member of the International Evidence-Based Guidelines on Minimally Invasive Pancreas Resection (IG-MIPR) (Miami, FL – USA, March 18-19, 2019). On September 17-20, 2023, Professor Boggi organizes the first world consensus conference on the treatment of borderline resectable and locally advanced pancreatic cancer (September 17-18, 2023). Professor Boggi is the founding chairman of the Italian Registry for Minimally Invasive Pancreatic Surgery (IGOMIPS), and a founding member of the European Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS), and of the International Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS).
BORDERLINE RESECTABLE PANCREATIC CANCER and LOCALLY ADVANCED PANCREATIC CANCER
We have radically removed pancreatic tumors that had been deemed unresectable by many national and international centers. This is why international patients with these tumors seek surgery in our center. Professor Boggi has personally performed over 2,200 pancreatic resections and has resected and reconstructed peripancreatic vessels (approx. 1000 vascular segments) in over >750 patients. This is likely to be largest, single-surgeon, world experience with these extended procedures. For sure, Professor Boggi has resected and reconstructed more superior mesenteric arteries than any other surgeon or center worldwide. Over the years the Pisa team has refined the technique for these formidable procedures. Results have improved. While the magnitude of these operations still carries a real risk of postoperative complications, if these resections are performed in patients who responded to chemotherapy, long-term results are encouraging. The International Scientific community recognized the value of pancreatic resections in both borderline resectable and locally advanced pancreatic cancer, that is now advised in medical guidelines. Therefore, patients with pancreatic tumors deemed unresectable solely because of local tumor growth (that in most patients means involvement of peripancreatic vessels) could apply to our center to verify if the tumor is truly unresectable. This decision is not taken only by surgeons, but rather by a large team of specialists including, medical oncologists, radio-oncologists, radiologists, endoscopy specialists/gastroenterologists, and pathologists. All specialists have a long experience in diagnosis and treatment of pancreatic tumor/disease, and most of them are dedicated to pancreatic cancer.
ROBOTIC PANCREATIC RESECTIONS
We have performed the first laparoscopic distal pancreatectomy at the end of the ‘90s and the first robotic pancreatoduodenectomy in 2008. Our group has pioneered minimally invasive surgery in many procedures such as: live donor nephrectomy (first case in Italy, April 27, 2000), laparoscopic live donor left lateral sectionectomy for adult-to-child liver transplantation (first case in Italy, May 2010), fully robotic right hepatectomy for adult-to-adult liver transplantation (first case in the World, April 17, 2012) (Transplant International 26 (Suppl. 2): 55-56), renal transplantation (first case in Europe; July 3, 2010) (Transpl Int. 2011 Feb;24(2):213-8. doi: 10.1111/j.1432-2277.2010.01191.x), pancreas transplantation (first case in the Wolrd; September 27, 2010) (Transplantation. 2012 Jan 27;93(2):201-6. doi: 10.1097/TP.0b013e318238daec), and distal selective spleno-renal shunt for minimally invasive treatment of severe portal hypertension (firs case in the World) (January 20, 2013) (Surgery. 2015 Feb;157(2):405. doi: 10.1016/j.surg.2014.07.012). We have now performed some 700 minimally invasive pancreatic resections, including >500 robotic pancreatic resections. No other center in Europe has performed as many robotic pancreatic resections. These operations, include >300 robotic pancreatoduodenectomies (so called “Whipple procedure”). We have described the feasibility of robotic Whipple (Br J Surg. 2013 Jun;100(7):917- 25. doi: 10.1002/bjs.9135), the possibility of adding vein resection (Langenbecks Arch Surg. 2016 Dec;401(8):1111-1122. doi: 10.1007/s00423-016-1499-8) as well as artery resection (Updates Surg. 2020 Mar;72(1):145-153. doi: 10.1007/s13304-020-00715-8). We also described how to improve the chance of radical resection for cancer of the head of the pancreas (so called “triangle Whipple”) (Surg Endosc. 2022 Jul 26. doi: 10.1007/s00464-022-09411-7). We have introduced robotic pancreatic surgery in many centers in Italy and Europe and we have taught several generations of Italian and International surgeons.
CONSULTATIONS
To have a preliminary consultation you can contact us at the following email address segreteria.profboggi@ao-pisa.toscana.it. If after a multidisciplinary evaluation our time believe that surgery could be an option in your case, further evaluation will probably require further investigations as well as at least one in-person visit. To have an initial evaluation of your case you should send a request to segreteria.profboggi@ao-pisa.toscana.it. You will be required to send a copy of all your medical exams and the tests. It will be important that you send a copy of images (not just reports) of all radiology investigations (such as computed tomography, magnetic resonance imaging, PER scan, etc). Ideally, you should send these exams on a CD (after having verified that it works properly) to the following address: Azienda Ospedaliero Universitaria Pisana Segreteria UO Chirurgia Generale e dei Trapianti Building n 6, 4th floor Via Paradisa 2 56124, Pisa, Italy If you cannot send these studies in a CD, you can try to transfer images through the web (e.g. wetranfer). It is important that you provide also all relevant information about previous oncology treatments (such as type, dose, and duration of chemotherapy regimens, dose of radiation therapy, last treatment received, etc.) as well as your past medical history (such as previous surgery, history of heart disease, medications, etc). It is also important that you send results of Ca 19.9 assays (as well as of other tumor markers, if available). To facilitate this, you can fill-in the electronic form that you can find at this link. Once we have all the information shown above, we can arrange for an online consultation. Unless you are covered by the Italian national health system (Sistema Sanitario Nazionale; SSN) (see below) you have to pay on your own for that. Payments go to Azienda Ospedaliero Universitaria Pisana, following a well-established pathway (your requested of online consultation will be forwarded to the offices of Azienda Ospedaliero Universitaria Pisana and the Hospital will take care of the payment, before the online consultation) As already introduced above, unless you are covered by the Italian national health system (Sistema Sanitario Nazionale; SSN), you are expected to cover the costs of all consultations, investigations, and treatments (including surgery). Costs can also be covered by a health insurance, but according to the rules set by our hospital (Azienda Ospedaliero Universitaria Pisana) you are expected to provide a money advance covering 90% of the anticipated expense. To define if you can afford that expense, you can require a cost estimate (again at: segreteria.profboggi@ao-pisa.toscana.it).
FAQ
1. What is a borderline resectable pancreatic cancer?
2. What is a locally advanced pancreatic cancer?
3. When a pancreatic cancer is “resectable”?
4. Why most surgeons deem locally advanced pancreatic cancer unresectable?
5. Do I need to receive chemotherapy before resection of a resectable pancreatic cancer or a locally advanced pancreatic cancer?
6. What are the risks of these extended pancreatic resections?
7. What are the long-term consequences of these extended pancreatic resections?
8. Can diabetes be prevented or mitigated in case of total pancreatectomy?
9. Why to consider a robotic pancreatic resection?
10. Who could have a robotic pancreatic resection?
1. What is a borderline resectable pancreatic cancer?
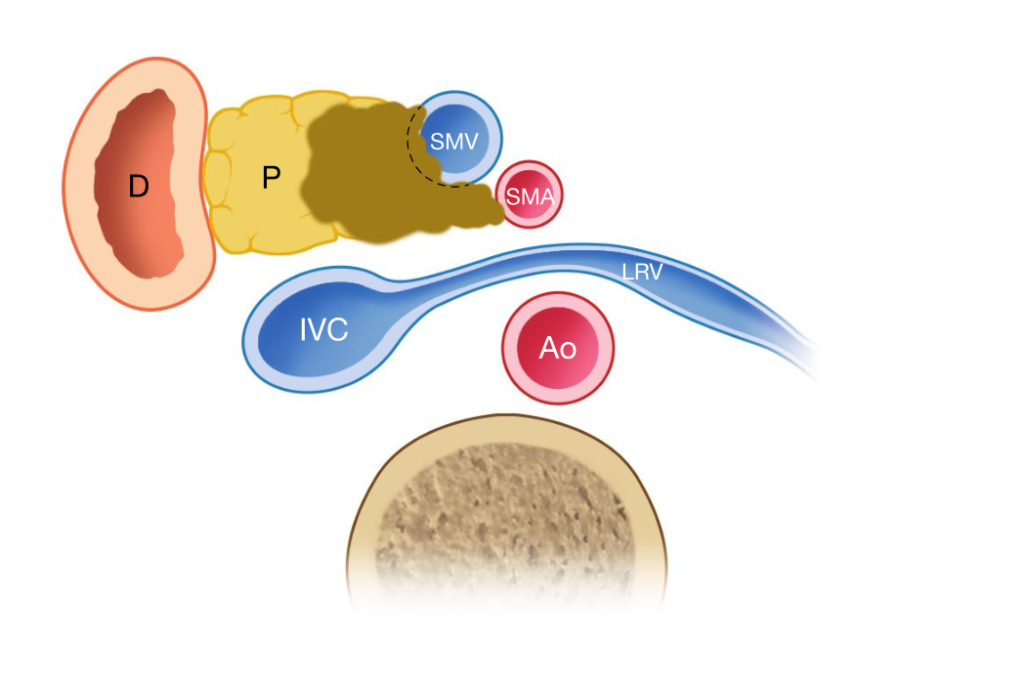
There are several definitions of borderline resectable pancreatic cancer. According to the National Comprehensive Cancer Network (NCCN) the following criteria define a borderline resectable pancreatic cancer: · Computed Tomography findings of venous distortion of the superior mesenteric/portal venous axis even including short-segment venous occlusion with proximal and distal sufficient vessel length allowing safe reconstruction; · Encasement of the gastroduodenal artery up to the hepatic artery, with either short- segment encasement or direct abutment of the hepatic artery without extension to the celiac axis · Tumor abutment of the superior mesenteric artery but with no greater than 180° of the vessel wall circumference. On practical grounds, most borderline resectable pancreatic cancers are tumors involving the superior mesenteric/portal vein. The tumor may be in contact with either the celiac trunk or the superior mesenteric artery but tumor abutment is <180° of the artery circumference (figure 1).
Figure 1. Example of resectable pancreatic cancer. (From Boggi U (Ed.) Minimally Invasive Surgery of the Pancreas. Springer-Verlag Italia s.r.l. 2018 – DOI: 10.1007/978-88-470-3958-2)
From a surgical point of view, a borderline resectable pancreatic cancer is a resectable tumor (i.e. it is technically possible to remove that tumor). However, upfront surgery (i.e. surgery before any oncology treatment) in borderline resectable pancreatic cancer has been associated with higher rate of microscopic margin positivity and lower rates of long-term survival (mostly due to the early development of occult metastasis at distant sites). Therefore, today, patients with borderline resectable pancreas cancer should receive preoperative chemotherapy (there is no clear evidence suggesting that they should also receive radiotherapy). Afterwards, resection can be considered basically if there is no evidence of distance metastasis (mostly in the liver, lung, and peritoneum), the patient is in good (ideally, perfect) shape, and Ca 19.9 levels (for those with initially elevated Ca 19.9 levels) have dropped. Tumor shrinkage may occur, but it is not routinely seen and it is not mandatory to permit surgery. More importantly, in most patients tumor downsizing is not so relevant that vein resection can be spared. Therefore, neoadjuvant chemotherapy or chemo- radiotherapy are not used with the purpose of sparing a vein resection and make (an already resectable) pancreatic tumor resectable (although this may be the explanation presented to most patients when chemotherapy is started). It is rather used to spare surgery in patients in whom initially occult metastasis become evident (grow) despite systemic medical therapy (i.e. chemotherapy). In patients with high levels of Ca 19.9, a decrease in this tumor marker demonstrates tumor response to chemotherapy and support the beneficial effects of tumor resection. However, some patients do not produce Ca 19.9.
What is a locally advanced pancreatic cancer?
According to the National Comprehensive Cancer Network (NCCN) the following criteria define a locally advanced pancreatic cancer: · Artery: encasement of the superior mesenteric artery or celiac axis, abutment or encasement of the first jejunal superior mesenteric artery branch, or abutment of the celiac axis and aortic involvement. · Vein: occlusion or tumor thrombosis of superior mesenteric vein or portal vein or abutment or encasement of the first jejunal superior mesenteric vein branch. Figure 2. Example of locally advanced pancreatic cancer. (From Boggi U (Ed.) Minimally Invasive Surgery of the Pancreas. Springer-Verlag Italia s.r.l. 2018 – DOI: 10.1007/978-88-470-3958-2)
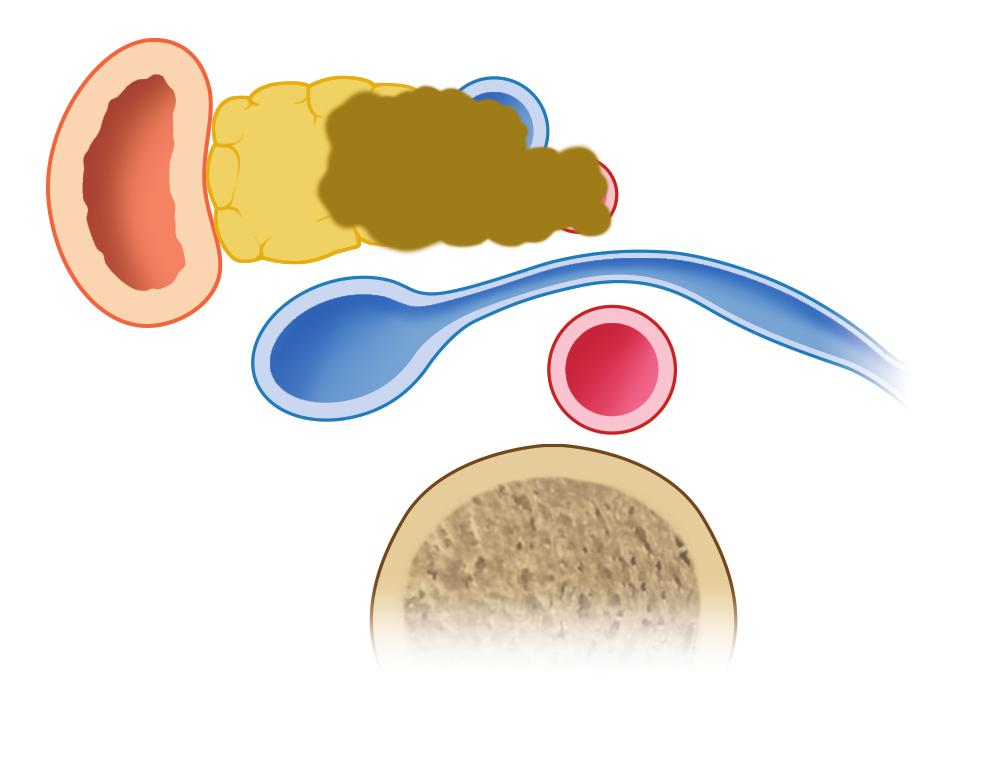
On practical grounds, most locally advanced pancreatic cancers involve both the superior mesenteric vein or portal vein and at least one large peripancreatic artery (i.e. the celiac trunk or the superior mesenteric artery). Traditionally this type of arterial involvement has been considered a sign of unresectability. However, recent evidence has shifted the concept on unresectable from anatomy (e.g. vascular involvement) to biology (e.g. response to oncological medical treatments). Nowadays, locally advanced pancreatic cancer should no longer be considered unresectable, if a positive response to chemotherapy or chemo-radiation therapy was observed: “Overall, we think that due to the advancements in neoadjuvant therapy as well as the technical advances in pancreatic surgery, the extension of the resectability criteria in locally advanced pancreatic cancer is reasonable. Surgeons and medical oncologists should be aware of the recent changes in NCCN guidelines and adjust their practice accordingly.” (Dimitrokallis N, Karachaliou GS, Moris D. New NCCN guidelines for locally advanced pancreatic cancer: new horizons in extending resectability. J BUON. 2020 Jul-Aug;25(4):2125-2126. PMID: 33099964.). The stage of pancreatic cancer is defined by the AJCC/UICC TNM staging system. That system is continuously revised according to emerging medical evidence. In the TNM, a locally advanced tumor basically corresponds to a T4 tumor (“Tumor involves the celiac axis, common hepatic artery or the superior mesenteric artery”). The TNM staging system does not even mention about the superior mesenteric/portal vein (further underscoring that vein involvement is not a barrier to resection). More importantly, in the last revision of the TNM staging system for pancreatic cancer (8th edition), a T4 pancreatic cancer is no longer defined unresectable.
8th Edition of the TNM staging system for pancreatic cancer
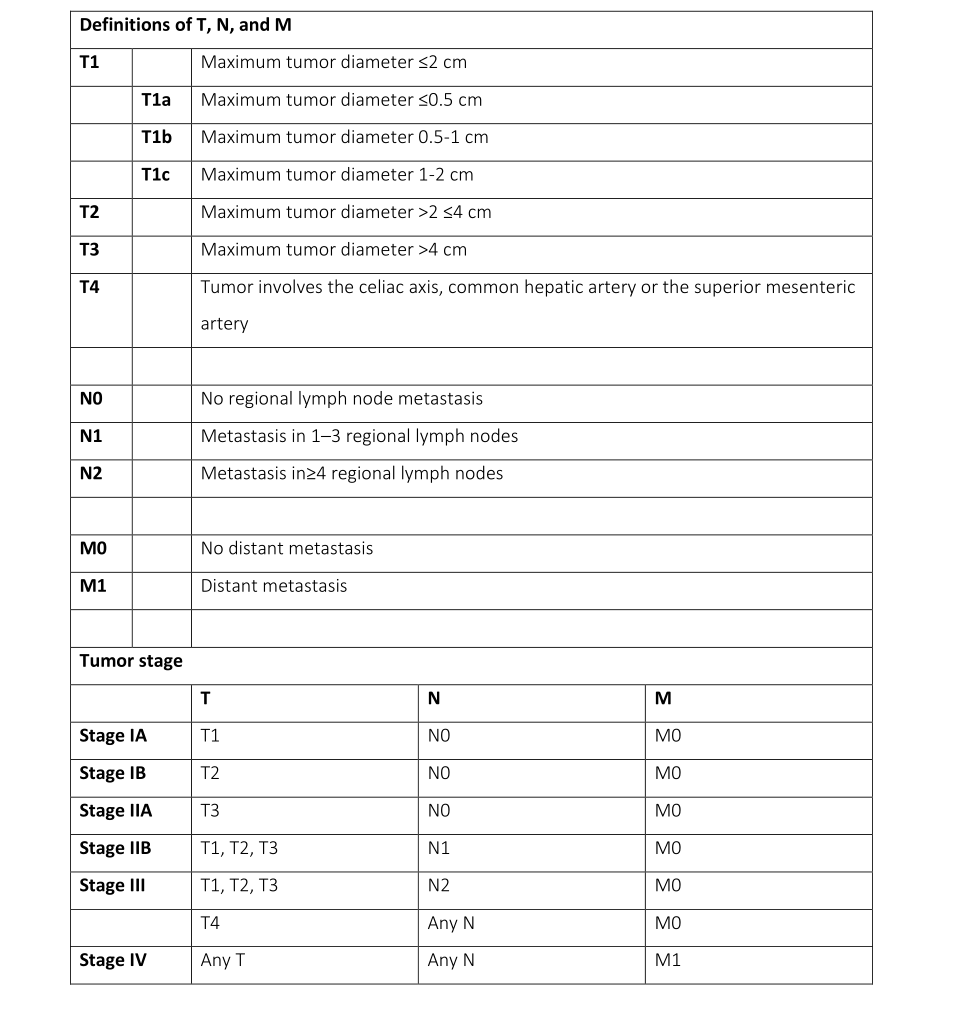
Definitions of T, N, and M T1 Maximum tumor diameter ≤2 cm T1a Maximum tumor diameter ≤0.5 cm T1b Maximum tumor diameter 0.5-1 cm T1c Maximum tumor diameter 1-2 cm T2 Maximum tumor diameter >2 ≤4 cm T3 Maximum tumor diameter >4 cm T4 Tumor involves the celiac axis, common hepatic artery or the superior mesenteric artery N0 No regional lymph node metastasis N1 Metastasis in 1–3 regional lymph nodes N2 Metastasis in≥4 regional lymph nodes M0 No distant metastasis M1 Distant metastasis Tumor stage T N M Stage IA T1 N0 M0 Stage IB T2 N0 M0 Stage IIA T3 N0 M0 Stage IIB T1, T2, T3 N1 M0 Stage III T1, T2, T3 N2 M0 T4 Any N M0 Stage IV Any T Any N M1 Our group has been among the few centers worldwide that have pioneered resection of locally advanced pancreatic cancer. We currently have one of the largest world experiences in pancreatectomy with arterial resection and the largest world experience in pancreatectomy with resection and reconstruction of the superior mesenteric artery (Boggi U, et al. Pancreatectomy with resection and reconstruction of the superior mesenteric artery. Br J Surg. 2023 Jul 17;110(8):901- 904. doi: 10.1093/bjs/znac363.). Although long-term survival remains basically unpredictable in the individual patient, but this is true also for those with seemingly early pancreatic tumors, we have now several patients who are alive and well (i.e. disease-free) 5 years and beyond after surgery. Our longest survivor is now alive and disease free 11 years after surgery. No other therapeutic approach can offer the chance of such long-term survival. In one of our recent publications, we have defined the factors that could predict long-term survival in these patients (Updates Surg. 2021 Feb;73(1):233-249. doi: 10.1007/s13304-020-00883-7).
When a pancreatic cancer is “resectable”?
Resectability does not just refer to the technical possibility of removing a tumor from the body. A modern view of resectability is based on the following parameters:
1. Absence of distant metastasis (such as liver, lung, or peritoneal metastasis)
2. The tumor can be removed from the body
3. The patient is able to undergo surgery with a reasonable risk of postoperative death (all types of pancreatic resections entail the risk of postoperative death). This means that the patient has to be in good general conditions (in technical terms: “fit for surgery”)
4. The patient is likely to have a survival benefit from surgery
Therefore, it is a combined definition. The most critical issue is to define when a patient is likely to benefit from resection. Actually, there is no agreed definition about when the benefits of surgery outweigh disadvantages (mostly in terms of operative risk and decreased quality of life). This difficult decision is taken by a multidisciplinary tumor board that provides a comprehensive evaluation of the patient, in the attempt to anticipate whether or not surgery will be “beneficial” for that individual patient. It is worth to emphasize that surgery may be beneficial also in “large” pancreatic tumors. All therapies other than surgery cannot truly offer the chance for long-term survival.
Why most surgeons deem locally advanced pancreatic cancer unresectable?
Most pancreatic surgeons have not received a formal training in vascular or abdominal transplant surgery (transplant surgery mostly revolves around managing and anastomosing visceral vessels). As a consequence, they simply do not feel comfortable in resecting and reconstructing large peripancreatic vessels. These surgeons sometimes ask for the collaboration of a vascular surgeon. Of course, vascular surgeons are able to anastomose vessels, but they are not familiar with visceral vessels and, more importantly, with the specific technical issues raised by an oncologic procedure and by the new anatomy that will be created following removal of the pancreas en-bloc with retroperitoneal soft tissues and vessels. Therefore, the ideal solution is to have everything in the hands of a single surgeon who has received specific training and is therefore able to manage all these complex issues at the same time.
Do I need to receive chemotherapy before resection of a borderline-resectable pancreatic cancer or a locally advanced pancreatic cancer?
Current National Comprehensive Cancer Network (NCCN) guidelines, as well all other oncological guidelines, state that before resection can be considered for borderline-resectable pancreatic cancer or a locally advanced pancreatic cancer patients must receive chemotherapy. Based on guidelines, these patients could also receive radiation therapy, although there is no clear evidence that it will further improve survival following tumor resection. Following chemotherapy (or chemo-radiotherapy) patients are evaluated by a multidisciplinary tumor board that should basically decide if tumor resection is possible and beneficial for that patient. This evaluation is based on current evidence and medical guidelines, but survival cannot be precisely anticipated in the individual patient (it can be anticipated in the average patient showing that characteristic).
What are the risks of these extended pancreatic resections?
On an average, standard pancreatoduodenectomy has a risk of postoperative mortality that is nearly 2 to 3-fold higher that that of open heart surgery. Therefore, all pancreatic resections are risky procedures. None can claim to be able to consistently prevent postoperative death in all patients undergoing pancreatic resection. The few series that have reported zero mortality, were based on a limited number of highly selected patients. Pancreatectomy with vein resection, and even more with arterial (± vein) resection, increases operative risk when compared to standard resections. Historically, in pancreatectomy with arterial (± venous) resection the risk of postoperative death has been exceeded 10%. In the last 10 years the risk has decreased. Now it is approximately 5% (1 postoperative death out of 20 operations). The risk of death is influenced by individual patient characteristic (such as age and associated diseases) and type of procedure.
What are the long-term consequences of these extended pancreatic resections?
In the majority, but not in all patients, requiring a pancreatectomy with arterial and venous resection, a total pancreatectomy is performed. In case of total pancreatectomy the obvious consequence of complete pancreas removal is diabetes (some patients are already diabetics before surgery). The type of diabetes that follows total pancreatectomy is defined “brittle”, as it is marked by frequent variations in glucose levels in the blood (either high or low levels). This type of diabetes can be managed but requires insulin therapy, dietary interventions, and strict adherence to medical prescriptions. Nearly all the patients who undergo these extended procedures develop postoperative diarrhea that can be extremely difficult to manage. Diarrhea is a consequence of removal of the extrapancreatic nerve plexus that is always invaded in locally advanced pancreatic cancer and in nearly all patients with borderline-resectable pancreatic cancer. Exocrine insufficiency, resulting from either total pancreatectomy or chronic pancreatic duct obstruction leading to pancreatic atrophy, aggravates diarrhea resulting from autonomic denervation. The combination of diarrhea and exocrine insufficiency (i.e. absolute or relative lack of pancreatic enzymes) typically leads to postoperative malnutrition. Eventually, both diarrhea and malnutrition can be managed and the patient will find a “new balance”, but this will take approximately 6 months and frequent consultations with dedicated medical doctors and nutritionists.
Can diabetes be prevented or mitigated in case of total pancreatectomy?
If total pancreatectomy is required for oncological reasons (i.e. the tumor is too large to be removed by a partial pancreatectomy), diabetes cannot be avoided. On the other hand, when total pancreatectomy is required for technical reasons (i.e. in patients with tumor in the uncinated process of the pancreas but who have a seemingly normal pancreas in the body-tail) diabetes may sometimes be avoided by means of islet autotransplantation. Pancreatic islets, the cells producing insulin, can be isolated from the body-tail of the pancreas and, if adequate in number and viability, can be injected into the liver by a percutaneous approach the day after surgery. In some patients, islet autotransplantation totally prevents diabetes. In other patients, it may mitigate diabetes. The possibility of pursuing islet autotransplantation depends also on the “endocrine reserve” of the individual patient (i.e. by the amount of insulin that the pancreas is able to secrete). The higher the endocrine reserve the lower the possibility of developing brittle diabetes despite islet autotransplantation. This option must be evaluated in the individual patient and needs to be carefully discussed.
Why to consider a robotic pancreatic resection?
Robotic surgery enhances surgeon’s ability to perform minimally invasive procedures of high technical complexity. As such, robotic assistance is particularly rewarding in pancreatic surgery. In general, minimally invasive procedures are associated with several advantages when compared to open surgery. Traditional advantages of minimally invasive surgery include: lower pain, faster recovery, lower blood loss (and therefore lower need of blood transfusions). All these advantages are also seen in minimally invasive pancreatic resections. Recent evidence suggests that there might be an oncologic advantage (longer survival following robotic pancreatic resections as compared to open pancreatic resections) and lower rates of post-operative complications (especially lower rates of clinically relevant postoperative pancreatic fistula).
Who could have a robotic pancreatic resection?
Not all patients are suitable for a robotic pancreatic resection. Suitability must be defined in the individual patient. In general, the patient must be fit for surgery and for laparoscopy (robotic surgery is a variation of traditional laparoscopic surgery). Overweight or obese patients (males more than females) could not be ideal candidate for robotic pancreatic resections. Concerning the tumor, only few (extremely well selected) patients with locally advanced pancreatic tumors are suitable for a robotic pancreatic resection. Patients with borderline pancreatic tumors may be more suitable for robotic surgery, but indication must be confirmed in the individual patient. Instead, practically all patients with “anatomically resectable” pancreatic tumors are eligible for a robotic pancreatic resection. Despite we still lack a high level of evidence, it is likely that robotic pancreatic resection will soon become standard of care in patients anatomically resectable pancreatic tumors, who are fit for surgery and laparoscopy